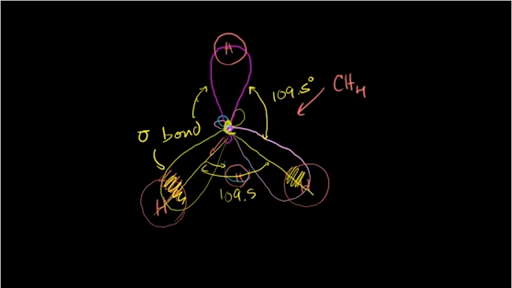
42 in the sketch of the structure of ch2br2 label all bonds
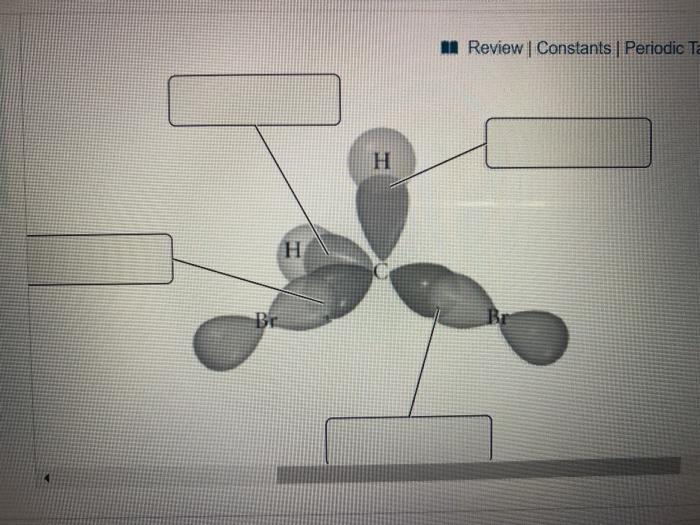
Solved Label all bonds in CH2Br2. Label all bonds in | Chegg.com Best Answer 92% (62 ratings) Transcribed image text: Label all bonds in CH2Br2 Label the diagram by dragging the labels to the appropriate targets. Note that not all labels will be used. o C (sp) H (s) o C (sp') Br (s) C (p) H (p) C (p) Br (p) C (sp) H (p) o C (sps) Br (p) C (sps) Br (p) reset help Previous question Next question Use valence bond theory to write the hybridization and ... - Socratic Step 1. Draw the Lewis structure (a) Start with a skeleton structure. The two #"C"# atoms (least electronegative) will be the central atoms, with the #"N"# attached to one of the carbons. (b) Attach the hydrogen atoms. The question gives you a clue where they go. The formula #"NCCH"_3# tells you that the three #"H"# atoms are attached to the terminal carbon atom.
Answered: In the sketch of the structure of BF3… | bartleby Transcribed Image Text: In the sketch of the structure of BF3 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Lone pair in sp orbital : B (p) - F (p) Empty p orbital Lone pair in p orbital B B (sp²) - F (p) в : В (8) — F (p) o : B (p) - F (p) Empty sp ...
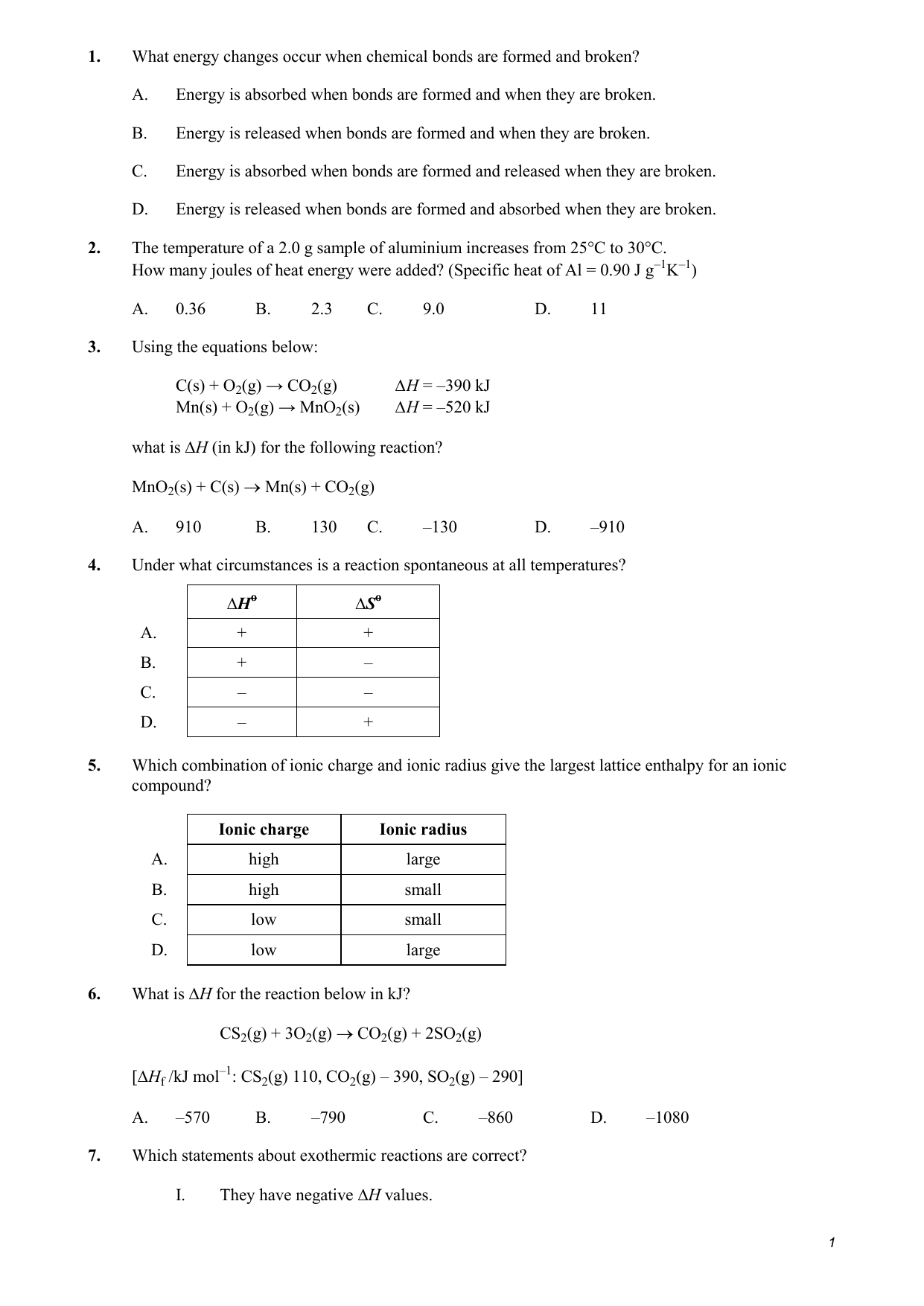
In the sketch of the structure of ch2br2 label all bonds
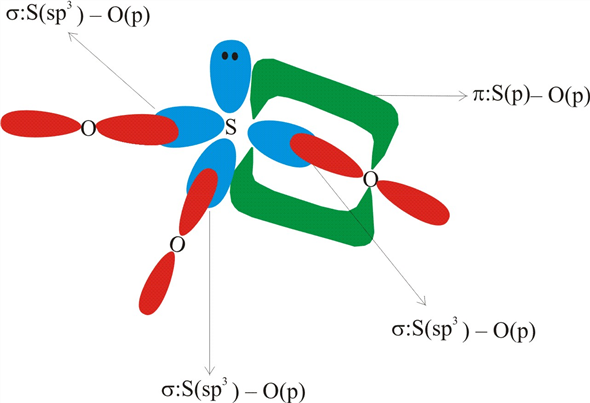
PDF Tro Chemistry - A Molecular Approach 2nd Edition Sketch the structure, cluding overlapping orbitals, and label all bonds using notation shown in Examples 10.6 and 10.7. combination of two Is orbitals. Indicate the region where inter- ference occurs and state the kind of interference (constructive or destructive). 70. Ch2br2 bonds - vkefsw.marcellogelato.pl In CH2Br2, C is the central atom. We are focusing on the hybridization of the central atom only. In the ground state, C has 2 unpaired electrons. It can only form two bonds. Promotion of electrons takes place, and all 4 valence electrons become unpaired. These 4 electrons are present in different orbitals. By bip39 mnemonic code converter Answered: In the sketch of the structure of SO2… | bartleby In the sketch of the structure of SO2 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. : S (p) - O (p) Lone pair in p orbital Lone pair in sp? orbital o : S (p) - O (sp²) т: S (p) — О (p) т: S (sp?)
In the sketch of the structure of ch2br2 label all bonds. Answered: Rank the free radicals (1-LII) shown… | bartleby Q: In the sketch of the structure of CH2B12 label all bonds. Drag the appropriate labels to their… A: All the bonds formed in CH2Br2 are sigma bonds. The hybridisation of C is sp3 Both, the C-Br bonds… Answered: Write a hybridization and bonding… | bartleby a. CH2Br2 b. SO2 c. NF3 d. BF3. Write a hybridization and bonding scheme for each molecule. Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in Examples 10.6 and 10.7. a. CH2Br2 b. SO2 c. NF3 d. CHEM: Chapter 10 Flashcards | Quizlet CH2Br2 b. SO2 c. NF3 d. BF3 Valence Bond Theory 76. Using the molecular orbital energy ordering for second-row homonuclear diatomic molecules in which the p2p orbitals lie at higher energy than the s2p, draw MO energy diagrams and predict the bond order in a molecule or ion with each number of total valence electrons. SOLVED:Write a hybridization and bonding scheme for each ... - Numerade Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in Examples 6.1 and 6.2. a. CH2Br2 b. SO2 c. NF3 d. BF3. Answer (a) See solution (b) See solution (c) See solution (d) See solution. View Answer. Related Courses. Chemistry 101.
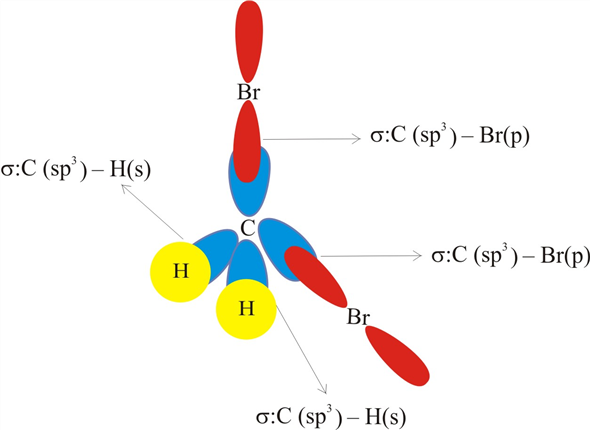
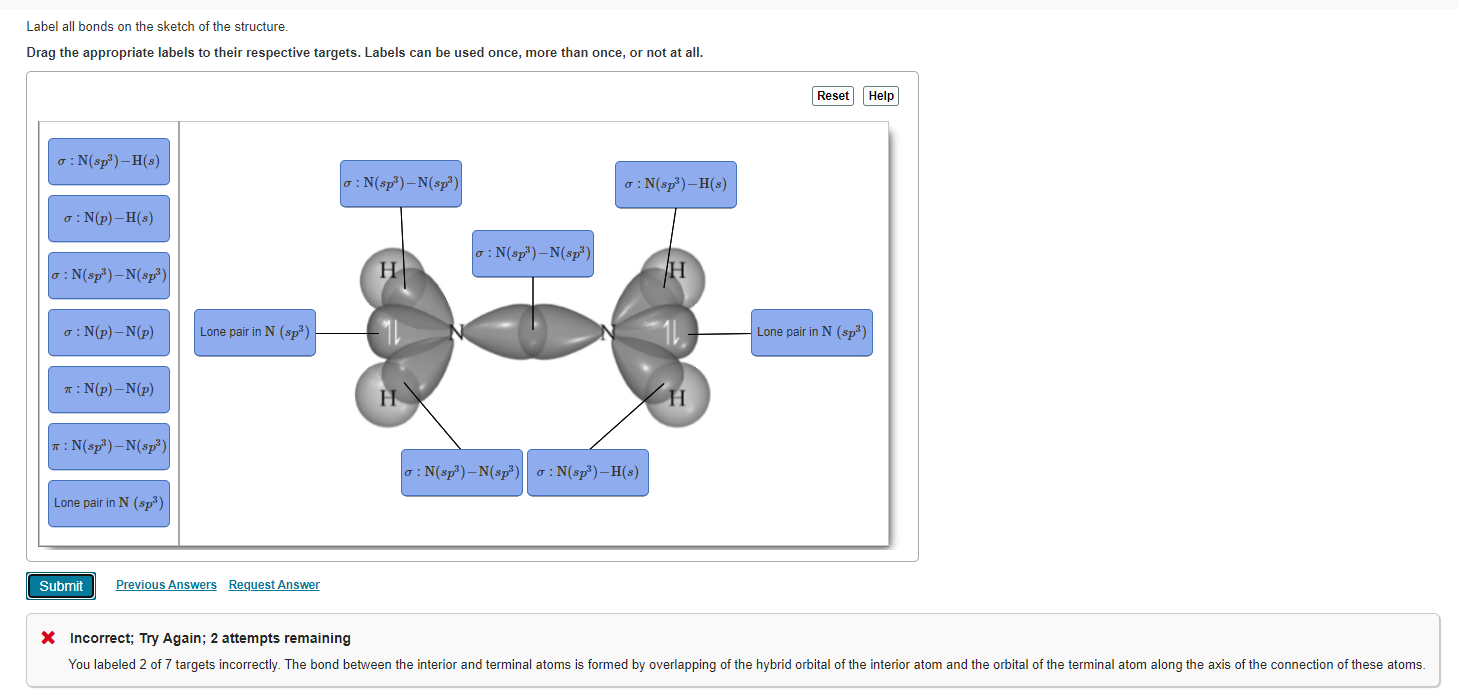
Chemistry: Semester 2, Unit 1 Practice Problems - Quizlet Sketch the structure, including overlapping orbitals, and label all bonds including the notation shown in examples 10.6 and 10.7 a. N2H2 (skeletal structure HNNH) b. N2H4 (skeletal structure H2NNH2) c. CH3NH2 (skeletal structure H3CNH2) ... 69: Sketch the bonding molecular orbital that results from the linear combinations of two 1s orbitals. Label all bonds in CH2Br2. Label all bonds in SO2. Label all bonds in ... Label all bonds in SO2. Label all bonds in NF3. Label all bonds in BF3. Answer The molecule's name is CH2Br Br Br br dibromomethane. The molecule can be described as a derivative methane. The central atom of carbon is bonded with two hydrogen atoms, and two bromine. All bonds are sigma. Below is the electron configuration for the atoms. OneClass: Label all bonds in CH2Br2? Get the detailed answer: Label all bonds in CH2Br2? Get the detailed answer: Label all bonds in CH2Br2? ... Chemistry: Structure and Properties. 2nd Edition, Tro. ISBN: 9780134293936. Chemistry: The Central Science. 14th Edition, 2017. Brown. ISBN: 9780134414232. Principles of Chemistry Molecular Approach. 4th Edition, Tro. Answered: In the sketch of the structure of… | bartleby Question Transcribed Image Text: In the sketch of the structure of CH2Br2 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all.
SOLVED:Write a hybridization and bonding scheme for each ... - Numerade Write a hybridization and bonding scheme for each molecule or ion. Sketch the structure, including overlapping orbitals, and label all bonds using the notation shown in Examples 6.1 and 6.2. a. COCl2 (carbon is the central atom) b. BrF5 c. XeF2 d. I3 Label all bonds in CH2Br2. Label all bonds in SO2. Label all bonds in ... Label all bonds in CH2Br2 Label the diagram by dragging the labels to the appropriate targets. Note that not all labels will be used. o C (sp) H (s) o C (sp') Br (s) C (p) H (p) C (p) Br (p) C (sp) H (p) o C (sps) Br (p) C (sps) Br (p) reset help Expert's Answer Solution.pdf Next Previous Q: View Answer Q: Q: Q: How many bonds are in CH2Br2? - Answers CH2Br2 is not an element, it is a compound of the three elements carbon, hydrogen, and bromine. It is formally known as dibromomethane. It is sometimes called methylene bromide. What type of isomer... Solved In the sketch of the structure of CH2 Br2 label all | Chegg.com Expert Answer 100% (14 ratings) Transcribed image text: In the sketch of the structure of CH2 Br2 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all.
Chapter 6, Chemical Bonding II Video Solutions, Chemistry: Structure ... Write a hybridization and bonding scheme for each molecule that contains more than one interior atom. Indicate the hybridization about each interior atom. Sketch the structure, including overlapping orbitals, and label all bonds using the notation shown in Examples 6.1 and 6.2. a. N2H2 (skeletal structure HNNH) b.
Post Lecture Lewis Structures, Problem Set #6 (Ch 5), Problem Set #7 ... Start studying Post Lecture Lewis Structures, Problem Set #6 (Ch 5), Problem Set #7 (Ch 6,7). Learn vocabulary, terms, and more with flashcards, games, and other study tools. ... Use the drawing of MO energy diagram to predict the bond order of Be2−. ... The molecule is polar because all the I−F bonds are polar and the net dipole moment is ...
Chapter 10: Chemical Bonding II: Molecular Shapes, Valence ... - Quizlet Begin by drawing the Lewis Structure (any resonance structure can be used to determine the number of electron groups) Based on the number of electron groups around the central atom, determine the geometry that minimizes the repulsions between electron groups ... Label all bonds using the σ or π notation followed by the type of overlapping ...
CH2Br2 Molecular Geometry - Science Education and Tutorials The formula of CH2Br2 molecular hybridization is as follows: No. Hyb of CH2Br2= N.A (C-H and C-Br bonds) + L.P (C) No. Hy of CH2Br2= the number of hybridizations of CH2Br2 Number of C-H and C-Br bonds = N.A (C-Br and C-H bonds) Lone pair on the central carbon atom = L.P (C) Calculation for hybridization number for CH2Br2 molecule
Answered: In the sketch of the structure of SO2… | bartleby In the sketch of the structure of SO2 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. : S (p) - O (p) Lone pair in p orbital Lone pair in sp? orbital o : S (p) - O (sp²) т: S (p) — О (p) т: S (sp?)
Ch2br2 bonds - vkefsw.marcellogelato.pl In CH2Br2, C is the central atom. We are focusing on the hybridization of the central atom only. In the ground state, C has 2 unpaired electrons. It can only form two bonds. Promotion of electrons takes place, and all 4 valence electrons become unpaired. These 4 electrons are present in different orbitals. By bip39 mnemonic code converter
PDF Tro Chemistry - A Molecular Approach 2nd Edition Sketch the structure, cluding overlapping orbitals, and label all bonds using notation shown in Examples 10.6 and 10.7. combination of two Is orbitals. Indicate the region where inter- ference occurs and state the kind of interference (constructive or destructive). 70.



















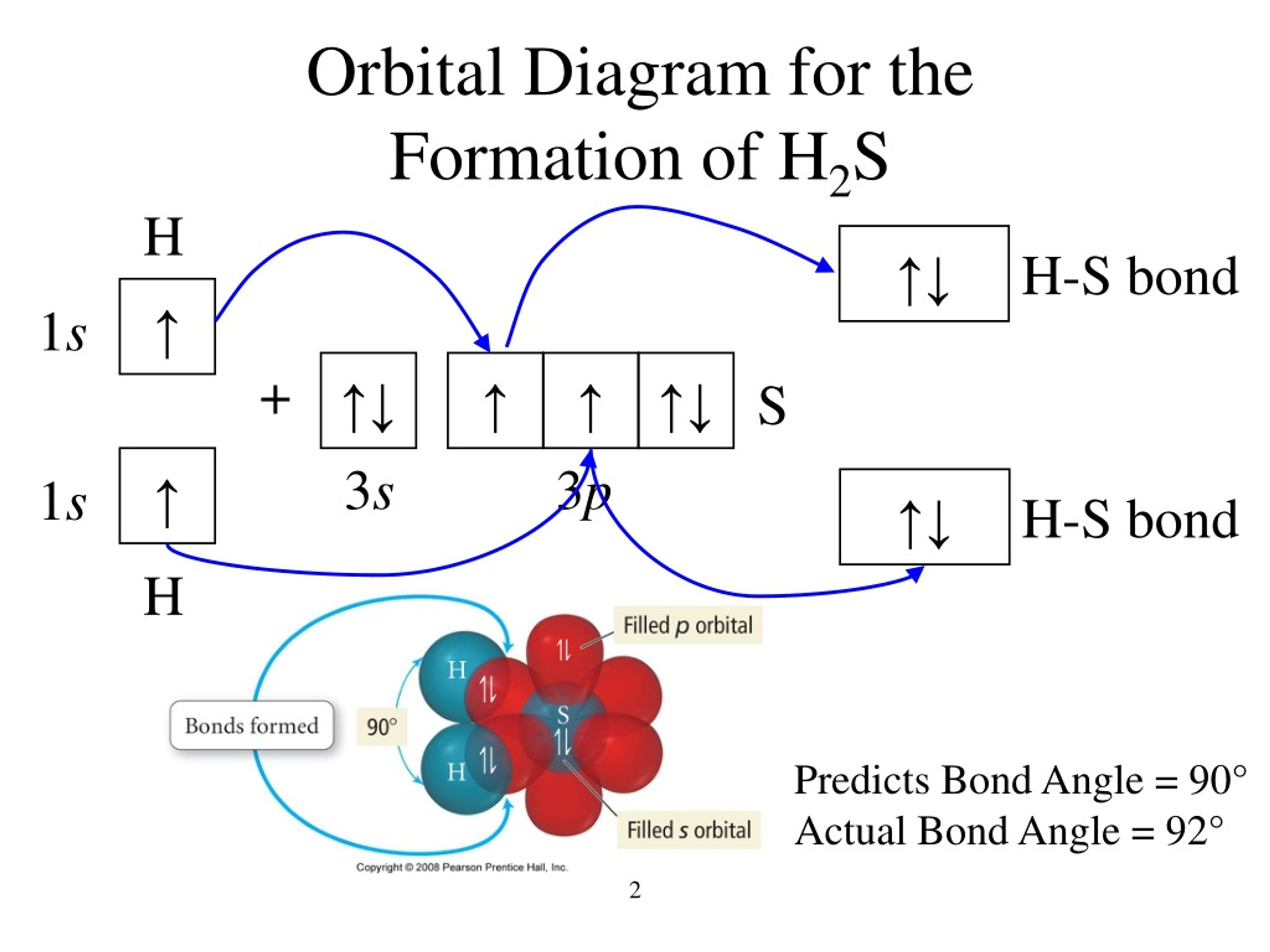
Post a Comment for "42 in the sketch of the structure of ch2br2 label all bonds"