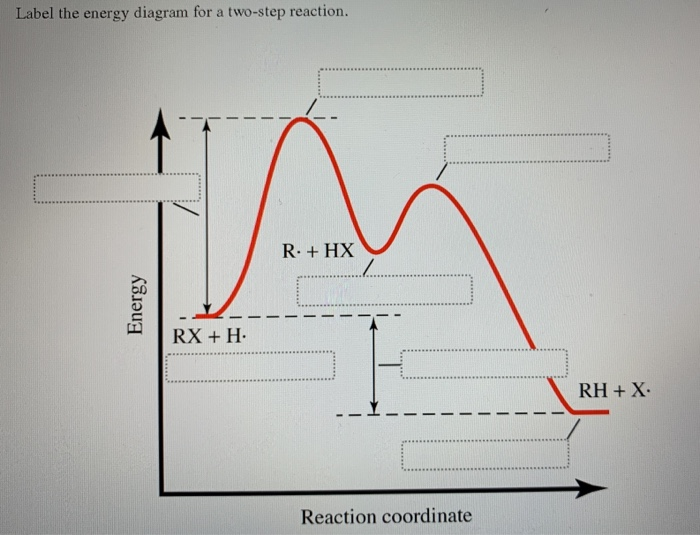
45 label the energy diagram for a two‐step reaction
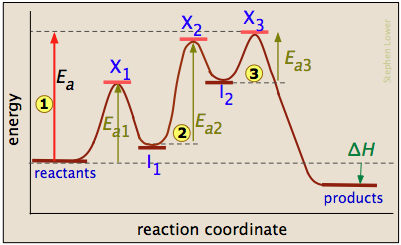
Multistep Reactions - Softschools.com The energy diagram of a two-step reaction is shown below. In the above reaction, a reactant goes through one elementary step with a lower activation energy (transition state 1) to form the intermediate. The intermediate then goes through a second step (transition state 2) with the highest energy barrier to form the product. Answered: Draw a potential energy diagram given… | bartleby Draw a potential energy diagram given the following information. - the reaction proceeds in three steps - the activation energy of step 2 is double that of step 1. - the activation energy of step 3 is double that of step 2 - the reaction is endothermic Label all axes, the rate limiting step, the location of any intermediates and transition states, the overall enthalpy of reaction, the ...
pubs.acs.org › doi › 10Graphene-Based Fiber Supercapacitors | Accounts of Materials ... Aug 01, 2022 · Energy density is a critical parameter in evaluating the electrochemical performance of SCs. The following equation can calculate the energy density of SCs: E = 1/2CV 2, where E represents the energy density, C is the capacitance, and V is the operating voltage window. On the basis of this equation, a 2-fold increase in voltage window could ...
Label the energy diagram for a two‐step reaction
Question #62891 | Socratic Explanation: The figure above represents the reaction profile of a two step, exothermic reaction. The y-axis represents the potential energy of the reaction species, and the x-axis represents the progress of the reaction. The reaction is exothermic because the energies of the products are lower than those of the reactants. Energy Diagrams: Describing Chemical Reactions Draw an energy diagram for a two-step reaction that is exothermic overall, and consists of a fast but endothermic first step, and a slow but exothermic second step. Indicate DGrxn, as well as DG1* and DG2* for the first and second activation energies, respectively. Label the positions corresponding to the transition states with an asterisk. Answered: Draw a reaction energy diagram for a… | bartleby Draw a reaction energy diagram for a two-step exothermic reaction whose second step is faster than the first step. Label the intermediate, transition states, ΔH, and activation energies.
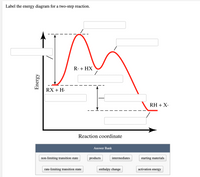
Label the energy diagram for a two‐step reaction. Draw an energy diagram for each reaction. Label the axes, the starting ... Draw an energy diagram for each reaction. Label the axes, the starting material, product, transition state, {eq}\Delta{/eq}H , and Ea. a. a concerted reaction with {eq}\Delta{/eq}H = -80 kJ/mol and Ea = 16 kJ/mol b. a two-step reaction, A -> B -> C, in which the relative energy of the compounds is A < C < B, and the step A -> B is rate ... Solved Label the energy diagram for a two-step reaction. - Chegg Expert Answer. 100% (54 ratings) Transcribed image text: Label the energy diagram for a two-step reaction. enthalpy change transition state starting materials RX+H products rate-limiting transition state intermediates activation energy reaction coordinate. How does the energy level diagram show this reaction is exothermic? Label ΔH as positive or negative. Figure shows the energy level diagram for the reaction between methane and oxygen. Based on Figure, the following information can be obtained. (a) The reaction between methane and oxygen to form carbon dioxide and water is an exothermic reaction. (b) During the reaction, the temperature of the mixture increases. How to draw the potential energy diagram for this reaction? 2. Identify the sign of the enthalpy change, ΔH, along with its value. The decrease in chemical potential energy should be the same as the amount of thermal energy released. That is. Ereactants − Eproducts = 2219.9lkJ⋅ mol−1. However, since. ΔH = Eproducts −Ereactants , ΔH = −2219.9lkJ⋅ mol−1. Note that by convention, the ...
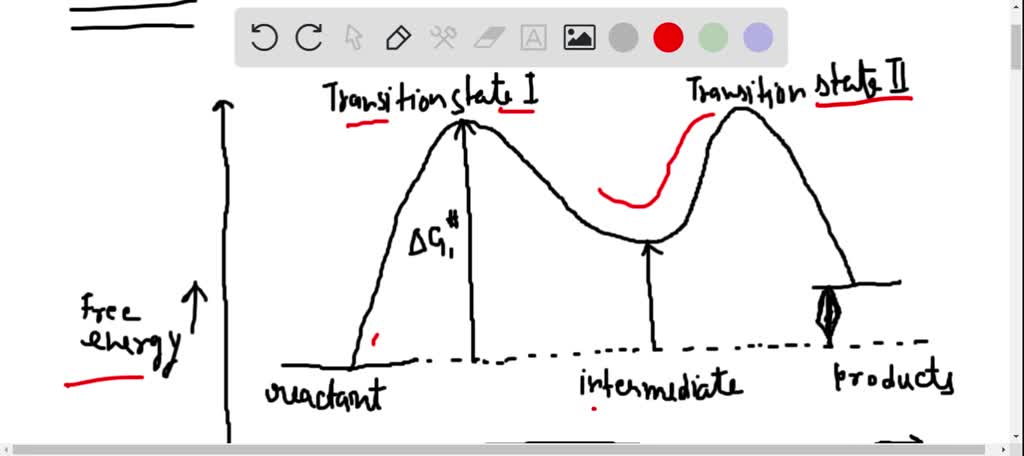
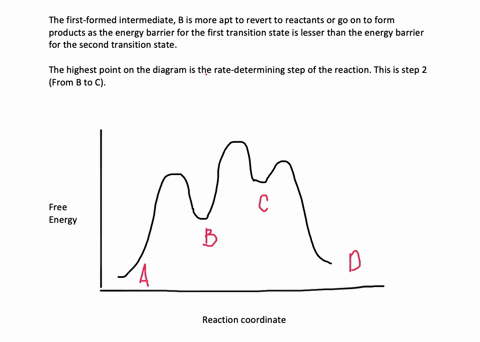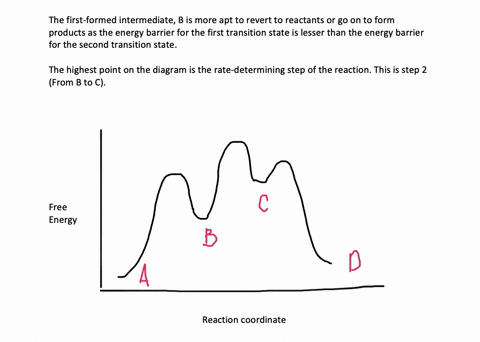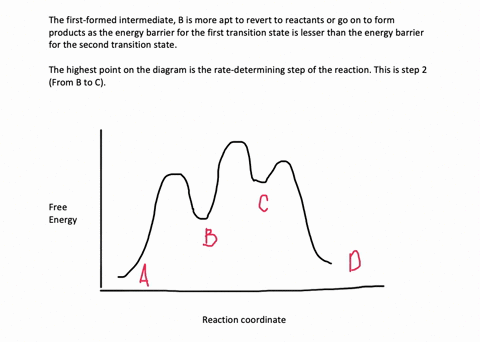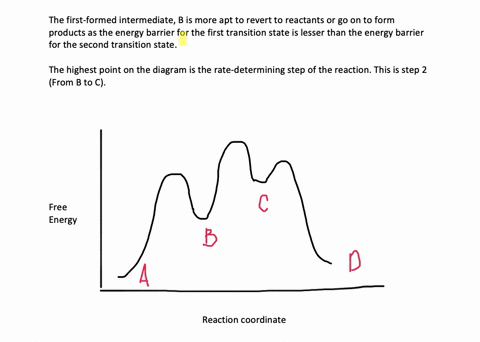
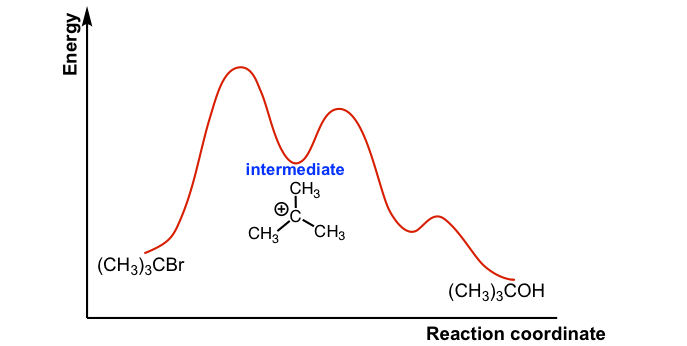
Energy Diagram for a Two-Step Reaction Mechanism Complete Energy Diagram for Two-Step Reaction A Two-Step Reaction Mechanism The transition states are located at energy maxima. The reactive intermediate B+ is located at an energy minimum. Each step has its own delta H and activation energy. The overall energy difference between the starting materials and products is delta H overall. Solved: Sketch an energy diagram for a two-step reaction in which ... Sketch an energy diagram for a two-step reaction in which both steps are exergonic and in which the second step has a higher-energy transition state than the first. Label the parts of the diagram corresponding to reactant, product, intermediate, overall Δ G‡, and overall Δ G °. Step-by-step solution 89% (9 ratings) for this solution Step 1 of 4 Draw an energy diagram for a two-step reaction where the first step is ... The reaction profile of a two-step reaction is represented by a potential energy diagram. The y-axis depicts the potential energy of the reacting species, while the x-axis depicts the reaction's progress. The first step of the reaction is said to be an exothermic reaction in which heat energy is released. › class › circlesRoller Coasters and Amusement Park Physics - Physics Classroom Determine the magnitude of any known forces and label on the free-body diagram. (For example, if the mass is given, then the F grav can be determined. And as another example, if there is no vertical acceleration, then it is known that the vertical forces or force components balance, allowing for the possible determination of one or more of the ...
Potential Energy Diagrams Answer-->. KNO 3 NH 4 Cl NH 4 NO 3 NaCl. 6/04. 21 A catalyst increases the rate of a chemical reaction by. (1) lowering the activation energy of the reaction (2) lowering the potential energy of the products. (3) raising the temperature of the reactants (4) raising the concentration of the reactants. Answer-->. Labeling an Energy Diagram Diagram | Quizlet Labeling an Energy Diagram. STUDY. Learn. Flashcards. Write. Spell. Test. PLAY. Match. Gravity. Created by. Corey_Williamson PLUS. Terms in this set (9) Reactants. Starting ingredients for Forward reaction. ... Potential Energy of Products for FORWARD reaction and Potential Energy of Reactants for REVERSE reaction. Analyzing Multi-step Reaction Energy Profiles - Study.com Analyze the following reaction energy profile: How many elementary steps are there? Step 1: Count the number of low points and high points in the diagram. There are two low points and three high ... Energy Diagrams of Two Step Reactions - YouTube Watch Complete videos @ Organic Chemistry 1
alex.state.al.us › plansALEX | Alabama Learning Exchange 11 ) Investigate different ways animals receive information through the senses, process that information, and respond to it in different ways (e.g., skunks lifting tails and spraying an odor when threatened, dogs moving ears when reacting to sound, snakes coiling or striking when sensing vibrations).
en.wikipedia.org › wiki › Cold_fusionCold fusion - Wikipedia Cold fusion is a hypothesized type of nuclear reaction that would occur at, or near, room temperature.It would contrast starkly with the "hot" fusion that is known to take place naturally within stars and artificially in hydrogen bombs and prototype fusion reactors under immense pressure and at temperatures of millions of degrees, and be distinguished from muon-catalyzed fusion.
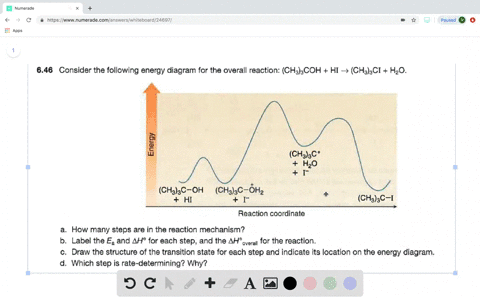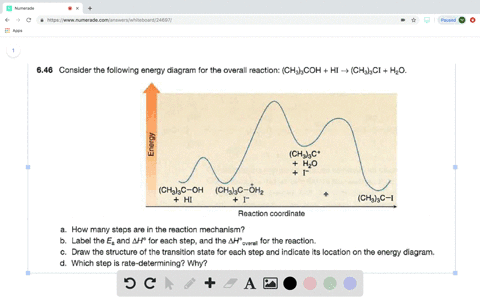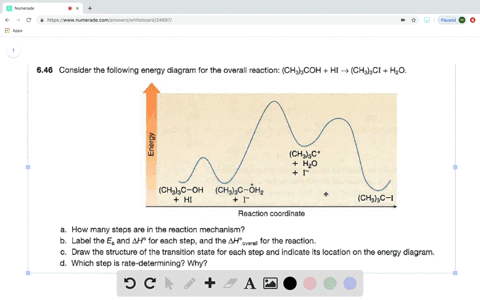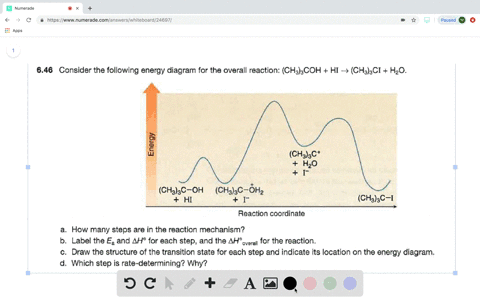
Answered: Consider the following energy diagram… | bartleby How many steps are present in the reaction mechanism? d. Label each step of the mechanism as endothermic or exothermic. e. Label the overall reaction as endothermic or exothermic. B G Reaction coordinate Energy ... Sketch an energy diagram for a two-step reaction in which both steps are exergonic and in which the ...
Energy Diagrams of Reactions | Fiveable Enthalpy, or heat energy, is represented by ΔH (Δ is the delta sign, which means change). If there is a negative change in energy, or -ΔH, an exothermic reaction is taking place and energy is released🔥 from the system to the surroundings. If there is a positive change in energy, or +ΔH, an endothermic reaction is taking place and energy is absorbed into the system from the surroundings.
microbenotes.com › krebs-cycleKrebs cycle / Citric acid cycle / TCA Cycle with steps and ... Mar 10, 2022 · This reaction is a reversible reaction catalyzed by the enzyme (aconitase). This reaction takes place by a two-step process where the first step involves dehydration of citrate to cis-aconitase, followed by the second step involving rehydration of cis-aconitase into isocitrate. Step 3: Oxidative decarboxylations of isocitrate
SOLVED:Draw a reaction coordinate diagram for a two-step ... - Numerade here for the reaction. Coordinate diagram. Put two step verification in which the first step, Andrea Janek, the second step is extra, Janek. And the order reaction is and a sonic labels are re intense. Products, intermediates and chronic transition is start stairs. So this is the reaction co ordination diagram in which this is for free energy, the bikes and X axis for the the reaction coordinates.
filestore.aqa.org.uk › sample-papers-and-markQuestion paper (A-level) : Paper 2 Organic and physical ... - AQA -diaminohexane by the reaction of 1,6-dibromohexane with an excess of ammonia. [2 marks] 0 1 . 2 Complete the mechanism for the reaction of ammonia with 6. form 1,6-bromohexylamine to -diaminohexane. Suggest the structure of a cyclic secondary amine that can be formed as a by-product in this reaction. [4 marks] Mechanism Cyclic secondary amine
Mechanisms and Potential Energy Diagrams - Course Hero The reaction whose potential energy diagram is shown in the figure is a two-step reaction. The activation energy for each step is labeled E a1 and E a2 . Each elementary step has its own activated complex, labeled AC 1 and AC 2 .
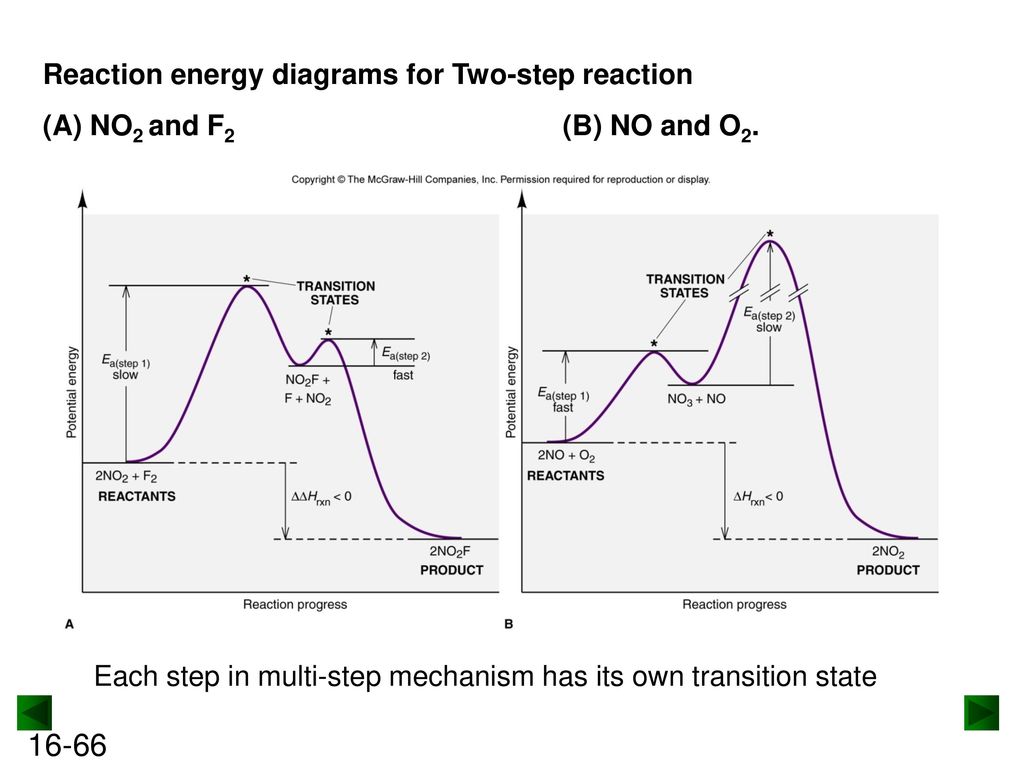
PDF cpb-ca-c1.wpmucdn.com Examine the following potential energy diagram and use it to construct a reaction mechanism. Show each step and the overall reaction. Label the rate-determining step. activation complexes aslc 2 Of F2 NOF + F Reaction Progress + 284 kJ/mol N02F Is DO is 00 Look at the PE diagram shown here and answers the questions below.
› solution-answer › chapter-19Chapter 19, Problem 65P | bartleby Ch. 19 - (II) Consider the following two-step process. Heat... Ch. 19 - (II) The PV diagram in Fig. 1931 shows two... Ch. 19 - (II) Suppose 2.60 mol of an ideal gas of volume V1... Ch. 19 - (II) In an engine, an almost ideal gas is... Ch. 19 - (II) One and one-half moles of an ideal monatomic... Ch. 19 - (II) Determine (a) the work done and (b ...
Draw an energy diagram for a two-step reaction, - Quizlet Find step-by-step Chemistry solutions and your answer to the following textbook question: Draw an energy diagram for a two-step reaction, $$ A \rightarrow B \rightarrow C, $$ where the relative energy of these compounds is C < A < B, and the conversion of $$ B \rightarrow C $$ is rate-determining..
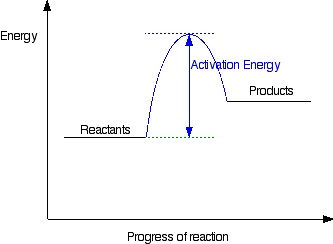
How can I draw activation energy in a diagram? | Socratic 1. Draw and label a pair of axes. Label the vertical axis "Potential Energy" and the horizontal axis "Reaction Coordinate". 2. Draw and label two short horizontal lines to mark the energies of the reactants and products. 3. Draw the energy level diagram. There must be a hump in the curve to represent the energy level of the activated complex. 4.
Answered: Draw a reaction energy diagram for a… | bartleby Draw a reaction energy diagram for a two-step exothermic reaction whose second step is faster than the first step. Label the intermediate, transition states, ΔH, and activation energies.
Energy Diagrams: Describing Chemical Reactions Draw an energy diagram for a two-step reaction that is exothermic overall, and consists of a fast but endothermic first step, and a slow but exothermic second step. Indicate DGrxn, as well as DG1* and DG2* for the first and second activation energies, respectively. Label the positions corresponding to the transition states with an asterisk.
Question #62891 | Socratic Explanation: The figure above represents the reaction profile of a two step, exothermic reaction. The y-axis represents the potential energy of the reaction species, and the x-axis represents the progress of the reaction. The reaction is exothermic because the energies of the products are lower than those of the reactants.



















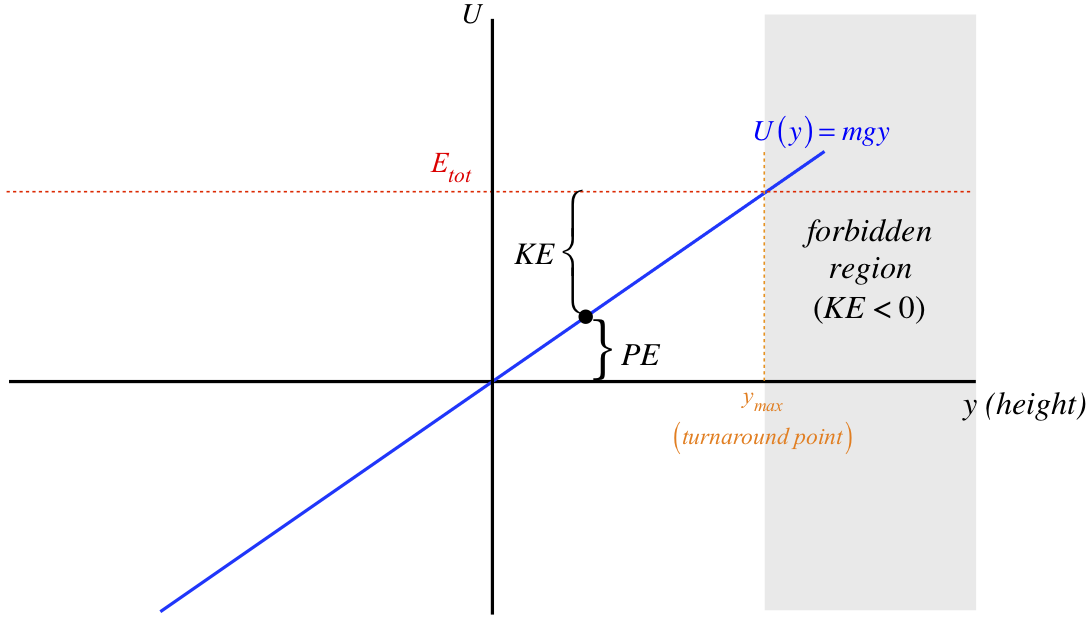

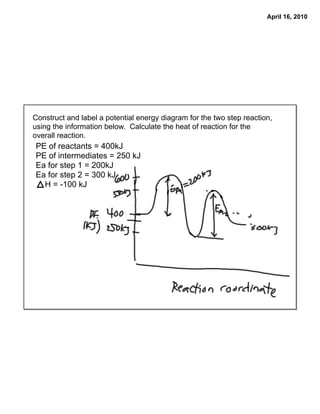
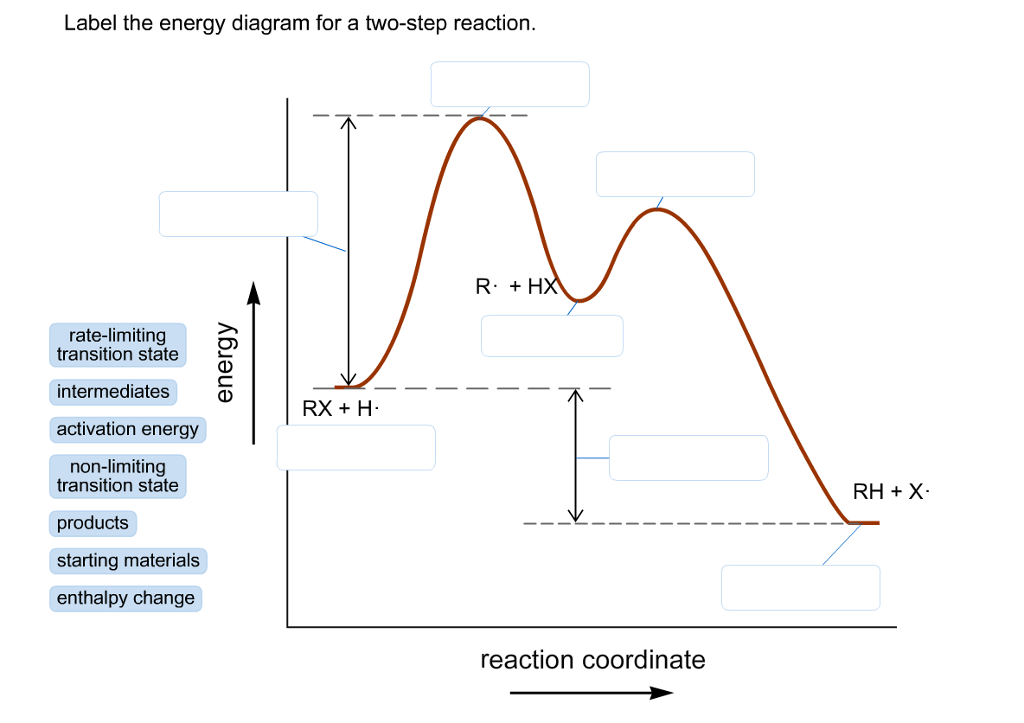
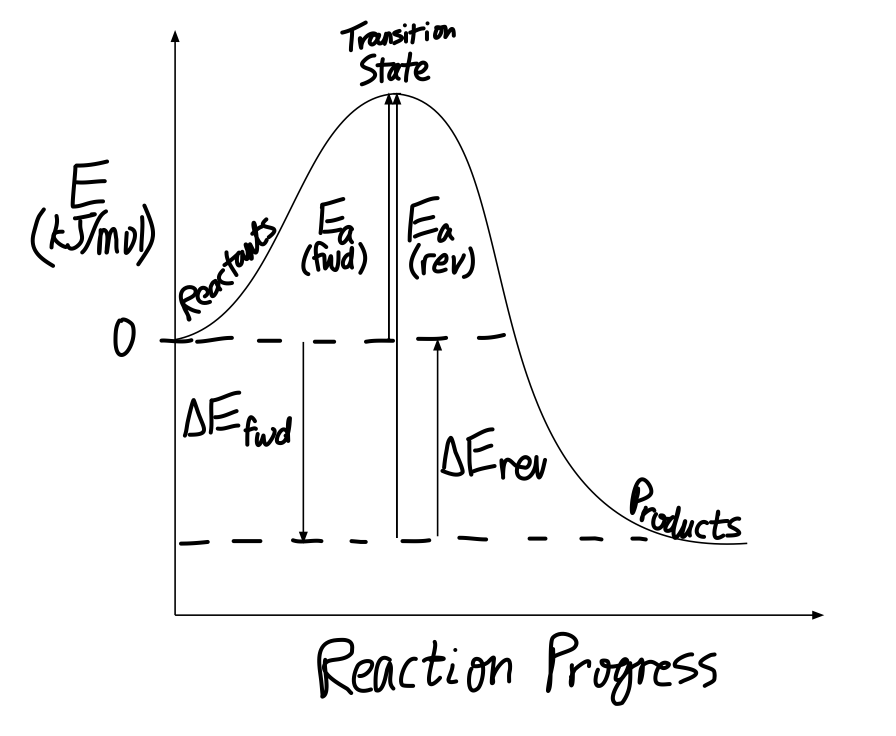
Post a Comment for "45 label the energy diagram for a two‐step reaction"